Plants have traditionally been understood as independent organisms with well-defined physiological limitations, particularly regarding the movement of minerals through their vascular systems. This understanding crystallized over centuries—from John Woodward's 1690s discovery that dissolved substances, not water alone, nourish plants, to Justus von Liebig's game changing agricultural chemistry in the 1840s. By the late 1800s, Julius von Sachs's aqueous culture experiments had mapped the behavior of individual nutrients within plant tissues, establishing what seemed to be immutable laws: certain minerals like nitrogen and phosphorus move freely through the phloem to where they're needed most, while others like calcium and manganese remain locked in place once deposited.
For over a century, this binary classification of mineral mobility has guided agricultural science and practice. Calcium deficiency will always manifest in new growth because calcium cannot be redistributed from older tissues, unlike mobile nutrients like nitrogen and potassium that move freely to where they're needed most. These patterns seemed as fixed as the plant's own anatomy, dictating everything from fertilization timing to deficiency diagnostics.
Plant nutrition science has long relied on this practical classification system that divides minerals based on their ability to move through phloem tissue—the plant's nutrient distribution highway. Mobile nutrients like nitrogen, phosphorus, potassium, and magnesium can be redistributed from older leaves to support new growth when supplies run low. This redistribution creates a predictable pattern: deficiency symptoms appear first in older tissues as the plant reallocates these nutrients to where they're needed most. In contrast, calcium, manganese, and certain micronutrients like boron have been classified as immobile or poorly mobile within the phloem. When these nutrients become scarce, deficiency symptoms emerge in new growth—the yellowing leaves, stunted shoots, and malformed fruits that signal inadequate mineral transport.
However, emerging research on the intricate relationships between plants and their endophytic microbiota—bacteria and fungi that colonize internal plant tissues without causing disease—contradicts these assumptions. These microbes appear to create alternative pathways that bypass the plant's inherent limitations. Like the discovery that mycorrhizal fungi extend root systems far beyond their physical boundaries, we're finding that endophytes may transform the very mechanisms of internal nutrient transport.
This research presents the potential for a systems-level transformation in how we understand plant nutrition, with far reaching implications for the future of agriculture with increasing resource constraints and continued pressure to produce an abundance of high quality crops.
The Hidden Network Inside Plants
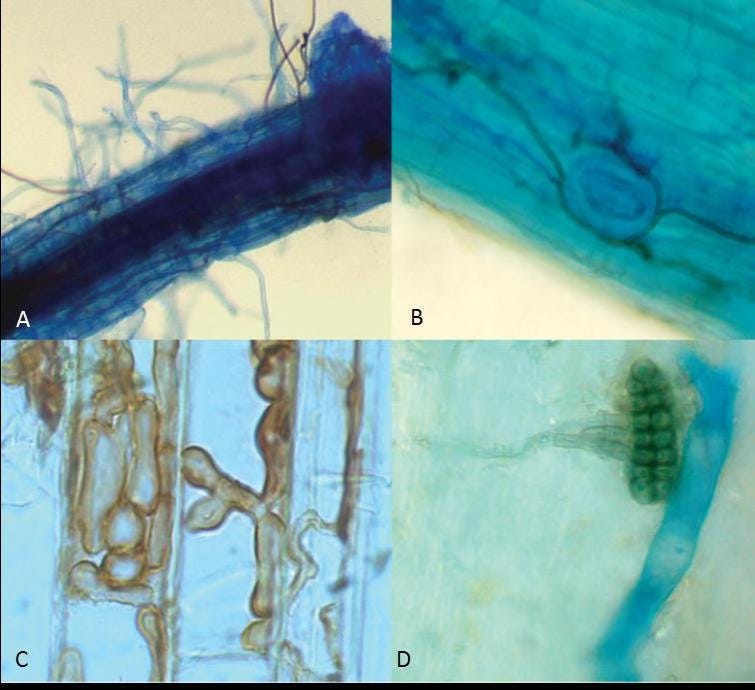
Recent advances in microscopy and molecular biology have unearthed that endophytic microbes establish far more extensive networks within plants than previously recognized. Dark septate fungal endophytes create a continuous integrated network that intimately interfaces with all sieve elements, cortical and epidermal cells. Meanwhile, bacterial endophytes ascend from roots to leaves through the vascular tissues of xylem and phloem, establishing colonies throughout the plant's internal architecture.
Within this sophisticated exchange system, plants provide their microbial partners with carbon compounds, primarily in the form of sucrose as confirmed by ¹³CO₂ tracing studies. In return, endophytes enhance nutrient acquisition, with documented increases in plant biomass of up to 100% compared to non-colonized plants. At the microscopic interfaces where fungal structures meet plant cells, researchers observe massive accumulations of lipids, indicating substantial material exchange between partners.
How Microbes Move "Immobile" Minerals
Three primary mechanisms may explain how endophytes facilitate movement of minerals:
Alternative Transport Networks
Fungal hyphae create continuous mycelial connections between different plant tissues, potentially establishing a secondary transport system. Unlike plant phloem, which operates under specific biochemical constraints, these fungal highways can move materials according to different principles. They interface directly with phloem sieve elements while extending throughout the plant, offering potential bypass routes for minerals that struggle to move through plant tissues alone.

Chemical Transformation of Nutrients
Endophytes produce an array of compounds that alter the chemical environment within plant tissues. They secrete indole-3-acetic acid and specialized molecules called phytosiderophores that significantly increase nutrient solubilization. For calcium specifically, endophytes improve intake by stimulating active transport through ion channels. By converting minerals from bound to bioavailable forms, endophytes may enable movement of nutrients that would otherwise remain fixed in place.
Modification of Plant Transport Systems
The presence of endophytes triggers substantial changes in plant physiology. Studies demonstrate that endophyte presence is closely tied to gene regulation and signaling changes that impact the plant's internal environment and behaviors. These changes extend to transport proteins, membrane characteristics, and vascular function—potentially enhancing the plant's own capacity to move nutrients.
Proof That Challenges the Textbooks
Multiple research findings directly contradict the established classification of certain minerals:
Calcium Transport
Research shows that endophyte fungi (Neotyphodium coenophialum) significantly increased the uptake and transport of phosphorus, potassium, calcium, and magnesium in tall fescue. This quantitative evidence of enhanced calcium transport in endophyte-colonized plants shows that mobility limitations may depend on the presence or absence of microbial partners. Further studies in wheat and barley demonstrated that calcium and manganese, typically considered to have low phloem mobility, were successfully remobilized from leaves, indicating conditional rather than absolute immobility.
Manganese Redistribution
The bacterium Bacillus sp. AP10 demonstrates how endophytes can enhance manganese mobility. Colonization increased the biomass, chlorophyll content, and translocation factor values of manganese in the aerial parts of host plants. Molecular analysis revealed that this enhancement occurred through regulation of genes responsible for the phenylpropanoid pathway and activation of ATP-binding cassette (ABC) transporter gene expression, particularly ABCB1, which is directly linked to enhanced manganese accumulation in plants.
The Precedent of Boron
The case of boron illustrates how our understanding of mineral mobility can evolve. Once classified as immobile, research has established that boron is mobile in all plant species that use simple sugars known as polyols as primary compounds in photosynthetic processes (peaches, apples, and berries among others). Boron forms complexes with these polyols, enabling transport in the phloem tissues to actively growing regions in the plant. This reclassification demonstrates how mechanistic understanding can transform established categories.
Implications for Agriculture
Understanding endophyte-mediated nutrient transport opens practical opportunities for improving crop production. If endophytes can mobilize nutrients internally, crops could better redistribute minerals, reducing the need for repeated fertilizer applications. This internal recycling could prove particularly valuable for calcium and micronutrient management in high-value crops.
Endophytes also produce mannitol, other carbohydrates, and small molecules like proline with antioxidant capacity, which help plants tolerate environmental stress. Combined with improved mineral nutrition, these compounds could enhance crop resilience to drought, heat, and other climate-related challenges. Additionally, enhanced mineral mobility could assist in phytoremediation efforts, allowing plants to more effectively extract and concentrate contaminants from agricultural soils.
Despite promising laboratory results, significant challenges remain in applying these findings to production agriculture. The relationship between plants and endophytes varies considerably among species and strains. Research emphasizes that the complexities of genetic factors involved in endophyte intervention strategies require further study, as different combinations may produce varying beneficial characteristics when tested on different model plants.
While evidence supports enhanced mineral mobility, researchers acknowledge that despite many documented instances of endophytes significantly affecting plant behaviors, there are often no direct and complete descriptions of the underlying mechanisms. Understanding these mechanisms at the molecular level is a crucial next step.
Laboratory successes don't always translate to field conditions. Recruiting beneficial endophytes in field applications is more complex than theory suggests because the intricate relationships between soil ecology and plants make introducing any microorganisms challenging. Successful colonization requires precise timing, with evidence showing that early colonization by endophytes tends to improve their sustained presence in mature plants.
The growing body of evidence on endophyte-plant interactions makes it clear that our understanding of mineral transport requires expansion beyond plant physiology alone. The plant-microbe system is an integrated unit, and should be viewed as such moving forward.
Diagnostic protocols will need revision to account for endophyte presence when assessing nutrient deficiencies. Fertility strategies could incorporate endophyte inoculation to enhance nutrient use efficiency. Plant breeding programs should select for traits that promote beneficial endophyte associations alongside yield and quality characteristics.
The phloem itself is also more complex than traditionally understood. It’s not only a sugar transport system but an integral signaling conduit carrying nucleic acids, peptides, proteins, hormones, and lipids. When combined with endophytic networks, this complexity has multiple potential pathways for mineral movement that we're only beginning to understand.
As agricultural systems face mounting pressure to produce more with less—less water, less fertilizer, less environmental impact—understanding and harnessing plant-microbe interactions becomes increasingly critical. Endophytes don't simply colonize plants; they alter how those plants acquire, transport, and utilize mineral nutrients. Recognizing and working with these relationships will prove essential for developing resilient, efficient agricultural systems capable of meeting our needs for an abundant and nutritionally rich food system.
Citations for Endophytic Microbes and Plant Mineral Transport
1. Historical Discoveries in Plant Nutrition Science
John Woodward's 1690s Discovery
Primary Source:
Woodward, J. (1699). Some Thoughts and Experiments Concerning Vegetation. Philosophical Transactions of the Royal Society of London, 21, 193-227.
Secondary Sources:
Eyles, V. A. (1971). John Woodward, F.R.S., F.R.C.P., M.D. (1665–1728): A Bio-bibliographical Account of His Life and Work. Journal of the Society for the Bibliography of Natural History, 5, 399-427.
Justus von Liebig's Agricultural Chemistry
Primary Sources:
Liebig, J. von (1840). Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie. Braunschweig: Friedrich Vieweg und Sohn. SCIRP +2
Liebig, J. von (1840). Chemistry in its Application to Agriculture and Physiology. London: Taylor and Walton. (English translation)
Secondary Sources:
Van der Ploeg, R. R., Bohm, W., & Kirkham, M. B. (1999). On the Origin of the Theory of Mineral Nutrition of Plants and the Law of the Minimum. Soil Science Society of America Journal, 63(5), 1055-1062.
Julius von Sachs's Aqueous Culture Experiments
Primary Sources:
Sachs, J. von (1865). Handbuch der Experimentalphysiologie der Pflanzen. Leipzig: Wilhelm Engelmann. Wikipedia +2
Sachs, J. von (1868). Lehrbuch der Botanik. Leipzig: Wilhelm Engelmann. Wikipedia +2
Modern Reviews:
Kutschera, U. (2015). Basic versus applied research: Julius Sachs (1832–1897) and the experimental physiology of plants. Plant Signaling & Behavior, 10(8), e1062199.
2. Traditional Classification of Mobile vs Immobile Nutrients
Binary Classification System
Jones, C., & Olson-Rutz, K. (2016). Plant Nutrition and Soil Fertility. Montana State University Extension Nutrient Management Module No. 2 (4449-2).
Michigan State University Extension Materials
Michigan State University Extension (2024). "Knowing nutrient mobility is helpful in diagnosing plant nutrient deficiencies." MSU ExtensionMSU Extension
Nutrient-Specific Classifications
Taiz, L., Zeiger, E., Møller, I. M., & Murphy, A. (2015). Plant Physiology and Development, 6th Edition. Sinauer Associates.
White, P. J., & Broadley, M. R. (2003). Calcium in plants. Annals of Botany, 92(4), 487-511.
3. Recent Research on Endophytic Microbes
Dark Septate Fungal Endophytes
Jumpponen, A., & Trappe, J. M. (1998). Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytologist, 140, 295-310. Wiley Online Library
Netherway, T., Bengtsson, J., Buegger, F., et al. (2024). Pervasive associations between dark septate endophytic fungi with tree root and soil microbiomes across Europe. Nature Communications, 15, 159.
Biomass Increases
Newsham, K. K. (2011). A meta-analysis of plant responses to dark septate root endophytes. New Phytologist, 190, 783-793. Wiley Online LibraryPubMed
Bacterial Endophytes and Vascular Transport
Olivares, F.L., et al. (2006). SHR5: a novel plant receptor kinase involved in plant–N2-fixing endophytic bacteria association. Journal of Experimental Botany, 57(3), 559-569.
Carbon Compound Exchange
Behie, S.W., Moreira, C., Sementchoukova, I., et al. (2017). Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nature Communications, 8, 14245. Naturenature
Lipid Accumulation
Jiang, Y. N., Wang, W. X., Xie, Q. J., et al. (2017). Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science, 356, 1172-1175.
4. Mechanisms of Enhanced Mineral Movement
Fungal Mycelial Networks
Fricker, M. D., Heaton, L. L., Jones, N. S., & Boddy, L. (2017). The mycelium as a network. Microbiology Spectrum, 5, FUNK-0033-2017.
Chemical Transformation
Chen, B., Luo, S., Wu, Y., et al. (2017). The Effects of the Endophytic Bacterium Pseudomonas fluorescens Sasm05 and IAA on the Plant Growth and Cadmium Uptake of Sedum alfredii Hance. Frontiers in Microbiology, 8, 2538. Frontiers +2
Nozoye, T., Nagasaka, S., Kobayashi, T., et al. (2011). Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. Journal of Biological Chemistry, 286, 5446-5454. NCBI
Gene Regulation
Sheibani-Tezerji, R., Rattei, T., Sessitsch, A., et al. (2015). Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. mBio, 6, e00621-15. PubMed Central
5. Evidence Challenging Traditional Classifications
Neotyphodium in Tall Fescue
Malinowski, D.P., Alloush, G.A., & Belesky, D.P. (2000). Leaf endophyte Neotyphodium coenophialum modifies mineral uptake in tall fescue. Plant and Soil, 227, 115-126. SpringerSpringer
Rahman, M.H., & Saiga, S. (2005). Endophytic fungi (Neotyphodium coenophialum) affect the growth and mineral uptake, transport and efficiency ratios in tall fescue (Festuca arundinacea). Plant and Soil, 272, 163-171. Springer
Calcium and Manganese Remobilization
Distelfeld, A., Avni, R., & Fischer, A.M. (2014). Senescence, nutrient remobilization, and yield in wheat and barley. Journal of Experimental Botany, 65(14), 3783-3798.
Bacillus and Manganese Mobility
Research from Ecotoxicology and Environmental Safety (2023) on "Endophytic Bacillus sp. AP10 harboured in Arabis paniculata mediates plant growth promotion and manganese detoxification" Taylor & Francis Online
ABC Transporter Expression
Jenness, M. K., Tayengwa, R., Murphy, A. S., et al. (2019). Loss of multiple ABCB auxin transporters recapitulates the major twisted dwarf 1 phenotypes in Arabidopsis thaliana. Frontiers in Plant Science, 10, 840260. Frontiers
Boron Mobility with Polyols
Brown, P.H., & Hu, H. (1996). Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol-rich species. Annals of Botany, 77, 497-505.
Stangoulis, J., Tate, M., Graham, R., et al. (2010). The mechanism of boron mobility in wheat and canola phloem. Plant Physiology, 153, 876-881.
6. Agricultural Applications
Stress Tolerance Compounds
Kamran, M., Imran, Q.M., Ahmed, M.B., et al. (2022). Endophyte-mediated stress tolerance in plants: A sustainable strategy to enhance resilience and assist crop improvement. Cells, 11(20), 3292. NCBIFrontiers
Phytoremediation
Ijaz, A., Imran, A., ul Haq, M.A., Khan, Q.M., & Afzal, M. (2016). Phytoremediation: recent advances in plant-endophytic synergistic interactions. Plant and Soil, 405, 179-195. Frontiers +3
Host-Microbe Specificity
White, J.F., Kingsley, K.L., Zhang, Q., et al. (2019). Review: Endophytic microbes and their potential applications in crop management. Pest Management Science, 75, 2558-2565. PubMed Central +3
Field Application Challenges
Hardoim, P.R., van Overbeek, L.S., Berg, G., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiology and Molecular Biology Reviews, 79, 293-320. ScienceDirect +2
7. Plant-Microbe System Integration
Phloem as Signaling Conduit
Kehr, J. (2006). Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. Journal of Experimental Botany, 57, 767-774.
Ruiz-Medrano, R., Xoconostle-Cazares, B., & Lucas, W.J. (1999). Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development, 126, 4405-4419.
Complex Soil-Plant-Microbe Interactions
Reynolds, H.L., Packer, A., Bever, J.D., & Clay, K. (2003). Grassroots ecology: plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology, 84, 2281-2291. Wiley Online LibraryASM Journals
Saleem, M., Hu, J., & Jousset, A. (2019). More than the sum of its parts: microbiome biodiversity as a driver of plant growth and soil health. Annual Review of Ecology, Evolution, and Systematics, 50, 145-168.




It’s not directly related, but this makes me think of a study on growing blueberries in grass in neutral soil (https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2019.00255/full). The common assumption is that grass can’t be near fruiting trees/bushes or it’ll compete too much for nutrients, but it may actually help make nutrients available for the plants and eliminate the need for artificial acidifiers that damage the existing soil.
It's wild, sometimes I feel like the entire world is just fungus wearing a planet costume.